|
|||||||||||
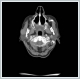
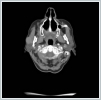
AbstractMantle cell lymphoma is a rare type of non-Hodgkin's lymphoma. First diagnose with parotid gland involvement is rare. An unusual case whose Mantle cell lymphoma diagnose obtained after parotidectomy was presented. Superficial parotidectomy was done with pleomorphic adenoma pre diagnosis and the pathological diagnosis was revealed as mantel cell lymphoma. To avoid unnecessary surgery of parotid masses, lymphoma should be considered as primary diagnosis.IntroductionHodgkin lymphoma’s include the lymph nodes primarily and only 5% include the extranodal sites, whereas 30% of non hodgkin lymphomas (NHL) existing in extranodal sites [1]. Mantle cell lymphoma (MCL) is an unusual lymphoma and it is roughly 5% to 7% of NHL’s [2]. Characterised by both the incurability of indolent lymphomas and the rapid growth of aggressive lymphomas, MCL has a median overall survival of only 4–5 years [2]. The participation of extranodal sites is not unusual as sufferers usually have an advanced-stage illness at the diagnosis [3]. In parotid masses, lymphoma’s are in differantial diagnosis. If there isn’a adequate lymph node for excitional biopsy, superficial parotidectomy must be done for definite diagnosis. In parotid gland mass’s we must always keep the lymphomas on mind by evaluating the tomograhy and fine needle aspiration biopsy to avoid an inessential surgery. We present this case on account of the rare pathologic result Mantle cell lymphoma from the parotid gland . Case ReportA 73-year-old woman suffered from a right parotid mass for one year. She had no pain, fever and other medical problems. There was no tobacco and alchol use. Computed Tomography imaging showed a 2 cm well defined mass in the right superficial parotid gland with homogeneous and strong enhancement (Figure 1).
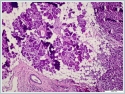
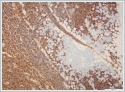
The mass was on the anterior of the gland and could not be clearly seperated from masseter muscle.The pre diagnosis of radioilgy was pleomorphic adenoma. In ultrasonography (USG) the mass was hypoecoic. A fine needle aspiration biopsy (FNAB) of her right parotid gland mass revealed benign cytology. Right superficial parotidectomy was done. After parotidectomy there was weakness in buccal branch of facial nerve but later it was normal. Pathologic examination revealed diffuse neoplastic lymphoid infiltration in salivary gland. (Figure 2)
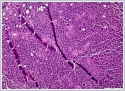
Neoplastic cells were small- to medium-sized monomorphic lymphoid cells with indented nuclear contours, dispersed chromatin, scant cytoplasm and inconspicuous nucleoli. (Figure 3)
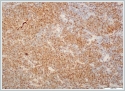
Immunohistochemicaly CD 20 (Figure4), CD5, cyclin D1 (Figure 5) and Bcl-2 were positive on neoplastic lymphoid tissue but CD23, CD10, CD3 were negative. Ki-67 index was %10 positive. The pathologic diagnosis was mantle cell lymphoma. Hematological values were hemoglobin 13,5 g/dl(11,2-15,7 g/dl), white blood cell count 4.210/mm[3](3.800-10.000/mm[3]) and platelet count 206.000/mm[3](150.000-350.000/mm[3]). Erythrocyte sedimentation rate was 9 mm/h. The patient's peripheral blood smear was normal. All biochemical parameters were normal. HbsAg, Anti-HCV, and anti-HIV tests were negative. On bone marrow aspiration and biopsy, there was minimal erythroid dysplasia. Dendritic cell marker CD11c expression was 65% on flow cytometric examination of bone marrow. Cytogenetic examination of bone marrow was normal. t(11;14) t(14;18) were not detected with fluorescence in situ hybridization (FISH) examination of bone marrow. But SOX-11 expression and p53 mutation could not be evaluated because of technical problems. Computed tomographies (CT) of thorax and cervical were normal. There were multiple lymphadenopathies on para-aortic and iliac regions on CT of abdomen. Positron emission tomography/computed tomography (PET/CT) could not be done, because our hospital had not PET/CT when diagnosed. After we used PET/CT at the end of treatment. According to Ann-Arbor staging [4], the patient were accepted as Stage II-AE. MIPI score (mantle cell lymphoma international prognostic index) [5] was 4 (intermadiate risk). The patient were treated with 6 cycles R-CHOP (rituximab, cyclophosphamide, adriamycine, vincristine, and prednisone) chemotherapy regimen. After 4 cycles, the patient was in partial remission. At the end of therapy, complete remission was achieved, after PET/CT examination. Now the patient is followed-up in complete remission. The patient’s informed consent is taken. DiscussionExtranodal lymphomas are seen almost specifically as NHL’s and these represent 10-20% of all lymphomas [6]. Inflammation of unknown source introducing as NHL of the head and neck may confirm to be a task for diagnosis as in our case [7]. Salivary glandular lymphomas are quite unusual and almost all them develop from B cells [8]. Patients with Sjögren's syndrome have an increased risk of NHL [9]. Lymphomas that are comman in Sjögren’s syndrome are usually extranodal low-grade B-cell NHL and the most seen type is marginal zone B-cell mucosa-associated lymphoid tissue type lymphoma (MALT) [10]. In our case sjögren’s syndrome was not detected. MALT is also the most seen lymphoma type in parotid glands [11,12]. Mantle cell lymphoma (MCL) is a rare type of NHL that presents for approximately 5% to 7% of them [2]. MCL’s are unusual and aggressive form of lymphomas [2]. In the last, it has been known as with various titles such as intermediately differentiated lymphocytic lymphoma, centrocytic lymphoma, and mantle zone lymphoma [2]. MCL occurs in middle-aged to older individuals with an average age of 60 years, and primarily in men [3]. According to Argatoff et al., the separated extranodal place of the illness has been known in 25% of cases, whereas salivary glands are hardly ever impacted (only 3% of revealed cases) [2]. In the same study of Argatoff et al. extranodal participation at demonstration happened in 76% of cases and the most typical sites were the bone marrow (63%), peripheral blood (34%), gastrointestinal tract (10%) and Waldeyer's ring (10%) [2]. In 25% of cases the extranodal location was the disease's main demonstration, while the most typical sites were the Waldeyer's ring (6%), intestine (5%), orbit (3%) and salivary gland (3%) [2]. The diagnosis of MCL is made on biopsies of lymph nodes, bone marrow or other affected tissues, or through blood examinations. In our case the MCL diagnosis was made with superficial parotidecomy. Parotidectomy operation is high in risk for facial nerve injury. Making this surgery only for diagnosis is not prefered. In our case before the operation lymphoma was not thought of diagnosis. Classical MCL exhibits a typical morphology of small- to medium-sized monomorphic lymphoid cells with indented nuclear contours, dispersed chromatin, scant cytoplasm and inconspicuous nucleoli. In contrast to follicular lymphoma , marginal zone B-cell lymphoma , lymphoplasmacytic lymphoma or small lymphocytic lymphoma / chronic lymphocytic leukaemia , blastic cells such as centroblasts, immunoblasts, or paraimmunoblasts, respectively, are not found in MCL. Besides classical MCL, there are four cytological variants, namely the small cell variant, the marginal zone-like variant, the pleomorphic variant and the blastoid variant Of these, the pleomorphic and blastoid variants are usually associated with an even poorer prognosis [13,14]. MCL has a attribute morphological overall look made up of small lymphoid cells with slightly irregular nuclear cells . Its histological growth designs are of a nodular or diffuse type, or a mixture of these two types [3,13]. In nodular MCL, some or many of the nodules may involve follicles with reactive germinal centers enclosed by broad mantles of small lymphoid cells or the mantle zone pattern. Our case was diffuse form. The immunohistological features of MCL reveal a characteristic phenotype. The cells express relatively extreme surface immunoglobulin M (IgM) and/or immunoglobulin D (IgD) and are usually positive for CD5, FMC7 and CD43, but negative for CD10 and BCL6. CD23 is negative or weakly positive. Almost all cases of MCL positive for cyclin D1. Usually, cyclin D1 is weakly positive in these cases. Cyclin D1 can also be indicated in plasma cell myeloma cases. In our case pathologic examination showed diffuse infiltration with neoplastic lymphoid cells. Lymphoid cells were typic histopathologic scene with small- to medium-sized monomorphic lymphoid cells. CD 20, CD5, cyclin D1 and Bcl-2 were positive but CD23, CD10, CD3 were negative . Ki-67 index was %10 positive. Cylin D1 positivty is seen in plasma cell myeloma and hairy cell leukemia . But there wasn’t any plasma cell differantion and immunohistochemicaly CD138 was negative. Hair cell leukemia is unusual for parotis and TRAP was negative . In the differential analysis on MRI; especially Warthin tumors and adenoid cystic carcinomas, with their strong cystic appearance must be thought of [15]. They present as well-circumscribed, partly cystic and partly solid lesions on MRI and are often located in the tail of the parotid gland. Adenoid cystic carcinoma’s are usually infiltrating masses with perineural invasion . On MRI adenoid cystic carcinoma has an irregular contour, poorly defined margin, and a strong contrast enhancement [15]. Lymphomas appear low in signal intensity on T1-weighted images and low to high but usually low in signal intensity on T2-weighted images [15]. In our case Computed Tomography imaging showed a 2 cm well defined mass in the right superficial parotid gland with homogeneous and strong enhancement. The radiologic initial diagnosis was pleomorphic adenoma. We did’nt want an MRI from the patient. Computed Tomography did not help to think lymphoma. If we could think the lymphoma pre diagnosis we could take an MRI. A similar case with parotid gland MCL was reported [16]. This case had prior a MCL diagnosis. After treatment a parotid gland was detected and her biopsy revealed MCL. In this case they didn’t need parotidectomy for diagnosis. But in our case FNAB revealed pleomorphic adenoma. If there isn’t an initially MCL diagnosis, the imagings can not be helpful in differential diagnosis and you need to do parotidectomy. Tt’s an important question how to avoid parotidectomy in such cases. We think this is the first case, MCL initially diagnosed with parotidectomy. Conclusion In conclusion by searching parotid gland masses, mantle cell lymphoma must be considered in the differential diagnosis. The parotid mass can be the first sign of lymphoma. References
|
|||||||||||
| Keywords : Mantle cell lenfoma , Parotis bezi , Non-Hodgkin lenfoma | |||||||||||
|