|
|||||||||
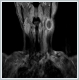
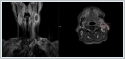
AbstractSpontaneous regression of primary malignant melanoma is characterized by a partial or complete disappearance of the neoplasm. Spontaneous regression of the primary site could be an explanation. But metastatic melanoma of unknown primary site (MUP) and spontaneous regression of melanoma metastases are a poorly understood condition. We present herein a patient with spontaneous regression of metastatic malignantant melanoma with unknown primary.IntroductionMetastatic malignant melanoma is a complex entity when it presents as a metastatic lesion with no primary site. It has been estimated that metastatic melanoma arising from an unknown primary site (MUP) accounts for approximately 1% to 8% of all melanomas [1]. These patients can be categorized into 2 clinical groups: those with metastatic involvement to lymph nodes only and those who present with nonlymph node disease. Various reasons for the primary being unknown have been suggested: occult cutaneous or visceral location for example anus, vagina or ENT; complete regression or primary origin in lymph nodes due to malignant transformation of ectopic naevus cells [2,3]. Spontaneous regression of metastatic malignant melanoma is very rare and it is reported as only 0.23%. The most common sites for metastases to undergo regression are cutaneous or subcutaneous deposits, followed by lymph node involvement. Other less common sites include, in order, pulmonary, hepatic, cerebral and intestinal metastases [4]. Case ReportA 64-year-old female presented with a mass in the left side of the neck for 3 months which was rapidly increasing in size. On examination, there was a mass measuring 5×3×2 cm in size in the upper 1/3 of the sternocleudomastoid muscle which was nontender and semi-mobile. Magnetic resonance imaging of the head and neck revealed a cystic mass which showed peripheral contrast enhancement while the submandibular salivary gland was remarkable (Figure 1).
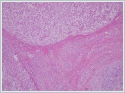
Fine needle aspiration cytology from this mass yielded necrotic cells but a specific diagnosis was not considered. We decided that surgical excision of the mass due to uclear diagnosis. It measured 4, 5×4, 5×2 cm with a smooth capsule and the cut surface showed large areas of haemorrhage and necrosis H&E stained sections from the tumor showed tumor cells arranged in the form of nests and lobules.(Figure 2)
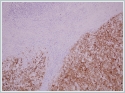
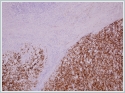
Few areas revealed brown- to black-colored pigment that stained positive for Masson’s Fontana, thus confirming that this pigment was melanin. Extensive sampling showed intracytoplasmic pigment in many cells. The tumor was strongly positive for S100, Melan A and HMB 45 (Figures 3A and 3B).
Malignant melanoma metastasis was detected as result of final histopathologic and immunohisto-chemical staining examination. There was no history of orbital enucleation or exenteration and history of excision of skin lesion. Complete clinical examination of the patient was carried out including all mucosal sites, skin and ocular examination to look for a possible primary site. A positron emission tomography (PET) scan was performed, which revealed multiple areas of likely metastatic spread. Several areas in the mediastinum and paracaval area were seen. The disease was explained to the patient, but the patient refused treatment. At this time, the patient began to live in rural. Her PET scan were repeated after 18 months showing complete resolution of her lymph node metastases. She now has no measurable disease and she describes herself as healthy. DiscussionMalignant melanoma is a potentially lethal neoplasm and a major public health concern. Melanoma of unknown primary and spontaneous regression of melanoma metastases are a poorly understood condition. Various theories have been developed concerning the appearance of the MUP phenomenon [1,5,6]. The first case proposed by Smith and Stehlinin in 1965 supported the disappearance of a primary lesion as a result of spontaneous regression after metastasis has occurred [7]. A second assumption was that the origin of the melanoma is in lymph nodes or other subcutaneous tissues or in viscera. Ectopic benign nevus cells have sometimes been found in lymph nodes and other tissues [8,9]. Third, a synchronous unrecognised melanoma and fourth possibility, a previously excised or traumatically removed melanoma have been proposed as explanations. The most likely explanation for MUP is immune induced regression of the primary tumour [10]. Melanoma accounts for 11% of all instances of spontaneous tumour regression. The incidence of spontaneous regression of metastases from malignant melanoma is approximately one per 400 patients, and possible mechanisms include operative trauma, infection, and immunologic. Other less well supported factors which have been associated with regression of metastatic melanoma include blood transfusion, BCG and rabies vaccinations, endocrine factors such as pregnancy or terminations, numerous alternative therapies, and conditions such as xerodersa pigmentosum, diabetes, nephrolithiasis, prostatic hypertrophy and peptic ulcers [4]. Indirect evidence for an immunologic response is found in the histologic features of regressing lesions that contain infiltrates of inflammatory cells, mainly lymphocytes [7]. We think that immunologic mechanism may have led to regression of both primary malignant melanoma lesion and its metastasis. Partial regression has been reported in 9–46% of primary melanomas. Complete spontaneous regression of metastatic melanoma is very rare, with an estimated incidence of 0.22–0.27%. Metastatic melanoma should be considered in the differential diagnosis of all patients who present with a malignancy of unknown origin, particularly when lymph nodes or cutaneous metastases are the primary presenting site [11]. Das Gupta criteria were described 45 years ago and they were mainly clinical ones [5]. But the end of the first decade of the 21st century new criteria should be evaluated with the introduction of imaging techniques as a mainstay for the diagnosis. Thorough clinical evaluation with total skin and subungual examination, detailed otorhinolaryngologic and eye evaluation with thorough oral and nasal mucosa assessment; proctoscopy, scrotal and gynecologic examinations for patients with inguinal lymph node metastases should be considered as appropriate. CT imaging of head and neck, brain imaging (CT or MRI preferably) and CT imaging of the chest/abdomen and pelvis should be escorted to rule out metastatic disease. Although PET/CT has a potential utility in the staging of melanoma, its role has not been demarcated for the identification of the primary site of MUP and therefore should not be recommended in first intent [12,13]. Spontaneous regression of melanoma metastases occurs equally in both sexes and in patients of all ages [4]. This contrasts with complete spontaneous regression of primary melanoma which has a male:female ratio of 2:1 [14]. The most common sites for metastases to undergo regression are cutaneous or subcutaneous deposits, followed by lymph node involvement. Other less common sites include, in order, pulmonary, hepatic, cerebral and intestinal metastases [4]. Spontaneous regression of metastatic lesions is probably associated with improved prognosis. There appears to be an increased immunological response by the host body against the tumour or specific metastatic deposits. Evidence for this is predominantly through histological examination of regressing tumours, mainly primary, but also metastatic lesions [15]. MUP metastatic to regional lymph nodes is more common in men than in women. It is also more likely to involve the axillary rather than the inguinal or cervical basin. The different distribution of melanoma primary in men and women may be an explanation. It is noteworthy that in men the metastases were located predominantly in the axillae, the head and neck region and the trunk, whereas in women metastases were found more frequently in the extremities. This is similar to the gender-related patterns of primary melanomas [16]. But in our case, malignant melanoma metastasis were in the neck. Conclusion Spontaneous regression of metastatic disease in melanoma appears to be stimulated by patients’ own immune responses. Targeting the host response through immunotherapy is the subject of many current trials and treatments with immune mediated solutions considered an area with significant potential. AcknowledgementWe acknowledge the contributions of Dr. Ozgur Erdogan for English translation support. References
|
|||||||||
| Keywords : Malign melanom , Primeri bilinmeyen , Spontan regresyon | |||||||||
|