|
|||||||||||
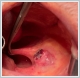
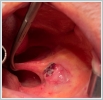
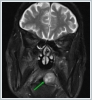
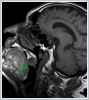
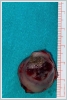
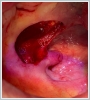
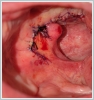
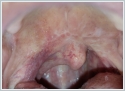
AbstractCanalicular adenoma is a benign neoplasia that originates mostly from minor salivary glands and accounts for approximately 1% to 3.2% of all salivary gland masses. About three-quarters of canalicular adenoma cases occur in the upper lip, but the buccal mucosa and hard palate are also frequently involved areas. It is most common in the seventh decade and is more common in women than men. Since the recurrence rate is very low when excised with clean surgical margins, it is important to differentiate it from malignant masses that require wider resection. Immunohistochemical tests are used to make this differential diagnosis. In this article, a case of soft palate canalicular adenoma with an ulcerated-crusted appearance, which was detected incidentally in a geriatric male patient who underwent cranial magnetic resonance examination for a different reason, and which is very rarely encountered in the literature, is presented.IntroductionCanalicular adenoma (CA) was first described in 1955 by Bhaskar and Weinmann [1]. It has been classified under the title of monomorphic adenoma by the World Health Organization for many years [2]. In previous studies, it was emphasized that the use of the term monomorphic adenoma should be avoided for adenomas in this group due to their different clinical and histopathological features and that they should be considered separately. In conclusion, CA has been accepted as a different salivary gland neoplasm with its clinical and histological features in the current histological classification of head and neck tumors by the World Health Organization [3]. CA is most common in the upper lip and is a benign neoplasm [3,4]. It represents 1% to 3.2% of all salivary gland masses [5,6]. It is commonly diagnosed in the 7th decade and is more common in women than men [7,8]. Recurrence is rare after excision with clean surgical margins [4]. In this article, a case of CA with isolated soft palate involvement and ulcerated-crusted appearance, which was detected incidentally in a geriatric male patient who underwent cranial magnetic resonance imaging (MRI) with the complaint of dizziness will be presented. Case ReportAn 82-year-old male patient was referred to our clinic because of a mass detected in his palate on a non-contrast cranial MRI performed in a secondary hospital to investigate the etiology of vertigo. The patient stated that he did not notice this lesion and did not have any complaints about his oropharynx. It was learned that he could not use the dental prosthesis for the last year because it did not fit his palate. The patient had no known additional disease and drug use. He had a history of cholecystectomy and appendectomy. On physical examination, a soft nodular lesion of approximately 2 cm in diameter, with an ulcerated crust superiorly, was observed in the left half of the soft palate (Figure 1).
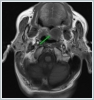
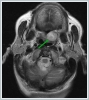
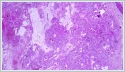
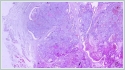
Informed consent was obtained from the patient. DiscussionMost minor salivary gland tumors develop on the palate, as minor salivary glands are common in this location [5]. The most common salivary gland tumor on the palate is pleomorphic adenoma [4]. It has been reported that 3% to 20% of the masses in the palate are CA [4]. CA is a rare, benign neoplasm that mostly originates from the minor salivary glands, may rarely develop in the parotid, and constitutes approximately 1% of all salivary gland masses [5]. In the study of Pires et al., this rate was determined as 3.2% [6]. Approximately 75% of CA cases are located on the upper lip, followed by the buccal mucosa and palate [3,4]. In the systematic review of Pereza et al., in which they examined 359 CA cases that evaluated the hard palate and the soft palate separately, 52 cases were detected in the hard palate, while there was only one case in the soft palate [8]. It is known that palatal CA occurs mostly at the junction of the hard palate and the soft palate. It is thought that the reason for this frequent location is due to the fact that the minor salivary glands located on the palate are frequent in this line [4]. CA is most common in the seventh decade [4,7,9]. It has been reported to be more common in females [7,9]. It usually presents as a well-circumscribed, painless, slow-growing nodular lesion, varying in size from a few millimeters to 3 cm, which may be solid, soft, or fluctuant on palpation [9,10]. Most of the patients present with the complaint of a painless mass. In the study of Thompson et al., 61 of 67 patients were admitted to the hospital with this complaint, while the mass was detected in 6 patients during examination [9]. Although ulceration may rarely be present in the tumor, bleeding, inflammation, pigmentation, and mucus extravasation may also ocur [5]. It has also been reported that it can be multifocal at a rate of approximately 20% [4,11,12]. The features that make our case interesting are that the tumor is located on the soft palate and has an ulcerated-crusted surface. Differential diagnosis of CA with malignant tumors is important to prevent unnecessary aggressive resections [11]. CA consists of a loose connective tissue stroma and columnar cells and is usually surrounded by a thin fibrous capsule [8]. The cells have hyperchromatic and oval nuclei and moderately stained cytoplasm. Consistent with its benign character, mitosis is rare. It has a duct-like epithelial arrangement [13]. Due to the basaloid structure of tumor cells, the differential diagnosis of minor salivary gland CA with basal cell adenoma, mucocele, and pleomorphic adenoma, and parotid CAs with Whartin tumor and adenoid cystic carcinoma is required [11,12]. Immunohistochemical tests are used in the differential diagnosis. Most CA cases have strong-diffuse staining with pan-cytokeratin (pan-CK) and S-100 [9]. In addition, high positive staining results were observed for chromogranin, synaptophysin, E-cadherin, and PCNA in the study of Pereza et al [8]. Polymorphous low-grade adenocarcinomas are also important in the differential diagnosis of palate masses [14]. In polymorphous low-grade adenocarcinoma, intense expression of CK7 and vimentin is always observed, whereas CK13 is negative [12,14]. Myoepithelial markers (α-smooth muscle actin, muscle-specific actin, smooth muscle myosin heavy chain, calponin, desmin) are negative in CA, which is thought to originate from ductal luminal cells. This allows immunohistochemical differentiation of CA from basal cell adenoma as well as polymorphous low cell adenocarcinoma [9,12]. Tumor cells react strongly with epithelial markers including pan-CK, CK7, low molecular weight cytokeratin, CK903, EMA, and CK19 [9]. In addition, it has been suggested that vimentin may also be a positive marker in the study of Thompson et al. [9]. In our case, PanCK, vimentin, E-Cadherin, and S100 were detected as strongly positive in tumor tissue in accordance with the literature. Detailed information about the radiological features of CA is not available, since many cases in the literature did not require radiological examination. The incidental detection of the lesion in cranial MRI performed for a different reason in our case enabled us to contribute to the literature on CA's radiological features. On MRI, CA was observed as a well-contoured, nodular lesion thought to have a capsular structure, which was heterogeneously hyperintense in T2W series and hypointense in T1W series. Polymorphous low-grade adenocarcinoma appears as a heterogeneous mass with low signal intensity on MRI in T1W and T2W series. There is no contrast enhancement [15]. Pleomorphic adenoma may have medium or low signal intensity in T1W series and high signal intensity in T2W series [15,16]. However, this signal distribution varies according to the density of the myxoid and cellular composition of the tumor [16]. Therefore, we think that MRI may be useful in differentiating CA from polymorphic low-grade adenocarcinoma, but its role in differentiating it from plemorphic adenoma is limited due to its variable characteristics. Parvizi et al. prefer incisional biopsy for histopathological diagnosis before excision of the mass [17]. However, Pires et al. reported that they mostly preferred excisional biopsy because CA is usually a lesion with smooth borders [6]. In our case, it was also supported by MRI images that these lesions were well-defined and did not invade surrounding structures. Therefore, we preferred excisional biopsy after MRI. Recurrence is rare when the CA is excised with a clean surgical margin [4]. The recurrence rate after excision has been reported to be approximately 5%, and it is thought that these recurrences may be caused by multifocality [10]. No cases of malignant transformation, metastasis, or tumor-related death of CA have been reported in the literatüre [5]. Regular clinical follow-up is recommended after surgical removal of the CA [14]. In our case, we did not perform additional resections and continue close clinical follow-up of the patient, since the surgical margins were clean as a result of the histopathological examination. ConclusionCA is a rare salivary gland neoplasia and it should be kept in mind in the differential diagnosis of soft palate masses. They may remain asymptomatic for a long time and may not be noticed by the patient. An ulcerated appearance suggests malignant lesions can also be present in canalicular adenoma, as in our case. Therefore, it is important to distinguish between CA, which can be successfully treated with simple excision, and malignant lesions that require aggressive treatment. Preoperative MRI may be useful for differential diagnosis and prevention of unnecessary resections. Informed ConsentInformed consent was obtained from the patient.References
|
|||||||||||
| Keywords : Kanaliküler adenom , damak , magnetik rezonans inceleme , tümör | |||||||||||
|