|
|||||||||||
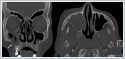
AbstractThe most common histopathology in sinonasal region malignancies is squamous cell carcinoma. Salivary gland tumors and lymphomas located in this region are extremely rare. In this article, three rare cases of malignancy affecting the palatomaxillary region are presented. These are non-Hodgkin lymphoma, adenoid cystic carcinoma and pleomorphic adenoma ex carcinoma cases. Radiologic imaging methods, diagnostic workups and treatment methods were discussed. Destructive lesions are seen in the computed tomography and magnetic resonance imaging in all the cases. Chemotherapy and radiotherapy were performed as a primary treatment for sinonasal lymphoma case. Surgical approach was the first treatment option for adenoid cystic carcinoma and pleomorphic adenoma ex carcinoma cases. Even if pre-operative punch biopsies yield benign results, the possibility of malignancy should be considered in large destructive lesions of this region. Postoperative radiotherapy and/or chemotherapy treatment may be required due to the anatomical difficulties of the region.IntroductionPrimary malignancies of the sinonasal region constitute less than 10% of head and neck cancers. Among these malignancies, squamous cell carcinoma is the most commonly encountered histopathology. They are followed by minor salivary gland tumors, sarcomas, esthesioneuroblastoma, lymphomas, undifferentiated tumors, and melanomas [1]. Maxillary cancers are rare and aggressive tumors [2]. These patients typically present at an advanced stage, leading to low survival rates. The spread of the disease and damage to the palatomaxillary region due to surgical treatments significantly impact the patient's quality of life [2]. Pleomorphic adenoma ex carcinoma is a malignant epithelial tumor of the salivary glands. It constitutes 2-4% of salivary gland tumors arising from pleomorphic adenoma [3-5]. The occurrence of pleomorphic adenoma in the maxillary sinus, apart from major salivary glands, is exceedingly rare. Pleomorphic adenoma ex carcinoma is extremely rare [3]. Sinonasal lymphomas are also very rare and may mimic benign processes such as rhinosinusitis. They may present as isolated lesions or with symptoms of systemic disease [6]. Histopathologically, diffuse large B-cell lymphoma is more common in lymphomas of the sinonasal region [6]. Adenoid cystic carcinomas are rare malignant tumors of major and minor salivary glands [7]. Adenoid cystic carcinomas are common in the hard palate in addition to the major salivary glands [8]. Adenoid cystic carcinomas are responsible for 5% of paranasal sinus malignancies. They grow slowly and are usually diagnosed at locally advanced stage [7]. Adenoid cystic carcinomas are slow-growing malignancies with a high local recurrence rate and are capable of distant metastasis [9]. In this article, three rare cases of malignancy affecting the palatomaxillary region are presented. These cases are non-Hodgkin's lymphoma, adenoid cystic carcinoma and pleomorphic adenoma ex carcinoma. Diagnostic difficulties and treatment approaches of these rare lesions are discussed in the light of the literature. Case ReportCase 1 A 66-year-old woman presented to our clinic with nasal obstruction for 3 months and swelling in the right canine premaxillary region for 10 days. On physical examination, a painful swelling was palpated in the canine region of the right cheek. Endoscopic endonasal examination was normal. Paranasal sinus computed tomography showed a millimetric defect in the upper wall of the right maxillary sinus at the level of the lower orbital wall (Figure 1).
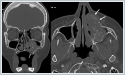
The patient was presented to the ENT Oncology council and referred to the Hematology clinic for treatment. There was no tumor in the 14th month follow-up of the patient who received chemoradiotherapy. Case 2 A 61-year-old woman presented to our clinic with nasal obstruction for 3 months. Endoscopic endonasal examination revealed a swelling on the lateral nasal wall at the level of the middle meatus on the left side, causing pushing into the nasal cavity. Paranasal sinus computed tomography showed a mass on the left lateral aspect of the maxillary bone extending into the left maxillary sinus and into the nasal passage at the level of the middle-lower meatus (Figure 3).
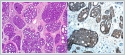
Left Caldwell-Luc approach combined with endoscopic endonasal approach was performed. It was observed that the anterior wall of the maxillary sinus was defective. It was observed that the mass started from the palatal portion of the maxillary bone and involved the entire maxillary sinus mucosa except the posterior portion of the sinus mucosa. The anterior, inferior and medial parts of the maxillary sinus were excised together with the mass. Intra-operative frozen examination was interpreted as benign. The final pathology result was reported as adenoid cystic carcinoma (Figure 4).
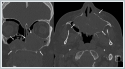
The patient was recommended maxillectomy because of residual tumor. The patient refused surgical treatment and was presented to the oncology council. Chemoradiotherapy was decided. There is no local recurrence in the 13th month of the treatment. Case 3 A 65-year-old woman presented to us with a long-standing complaint of nasal congestion. Endoscopic examination of the nasal cavity revealed a hard smooth circumscribed mass filling the left nasal cavity, invading towards the base of the septum and extending to the base of the right nasal cavity. Preoperative imaging showed a mass filling the left nasal cavity almost completely (especially the mid-posterior part), destructing the nasal septum and reaching the right nasal cavity, extending from the right nasal cavity to the postnasal region, thinning the medial bone wall of both maxillary sinuses, bowing and forming a defect, thinning the anterior wall of the left maxillary sinus, and causing pathological changes in the surrounding bone structures. In addition, it was reported that the mass significantly thinned the bony structures in the palatine bone and the alveolar part of the maxillary bone and formed defects in places, and also formed a defect in the bone in the left maxillary sinus inferiorly (Figure 5).
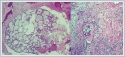
Incisional biopsy was performed twice from the lesion in the nasal cavity. The pathology results suggested sinonasal papilloma in the first case and was reported as pleomorphic adenoma in the second case. Bilateral Caldwell-Luc and endoscopic endonasal approaches were combined. A mass was seen in the left maxillary sinus. The mass invaded the palatal bone at the base of the nose. The tumor progressed submucosally on the nasal floor and extended to the opposite side under the septum. The tumor was observed to extend posteriorly under the vomer. It could not be removed unblocally due to the size and extensive invasion of the mass. At the base, the infiltrating necrotic areas of the palatal bone were excised and drilled. The palatal mucosa was found to be intact. Intraoperative frozen biopsy was interpreted as benign. The final histopathologic examination was reported as pleomorphic adenoma excarcinoma (high grade adenocarcinoma) (Figure 6).
The patient was presented to the Otorhinolaryngology Oncology council with the imaging results and the patient was recommended for a repeat operation after the residual lesion was seen in the imaging tests. With bilateral Caldwell-Luc approach, inferior nasal septum resection and partial hard palate resection was performed. In order to enable the patient to use an obturator prosthesis, the hard palate was excised together with the nasal floor septum inferior to the border of the soft palate posteriorly, leaving approximately 1 cm of gingival arch. No macroscopic tumor remained. Histopathologic examination was reported as pleomorphic adenoma ex carcinoma. Because of microscopic positive surgical margin at the soft palate border, chemoradiotherapy treatment was deemed appropriate. The patient died 9 months later due to pneumonia. DiscussionAs in other head and neck cancers, the most common malignancy of the sinonasal tract is squamous cell carcinoma. Minor salivary gland tumors, sarcomas, esthesioneuroblastoma, lymphomas, undifferentiated tumors and melanomas are less common [1]. Maxillary sinus cancers are rare and aggressive tumors that can spread to the sinus bone walls and surrounding tissues. Approximately 80% of maxillary sinus cancers are diagnosed at an advanced stage [2,10]. Patients may present with various symptoms. In a study investigating the symptoms and clinical findings of maxillary sinus malignant tumors (65.7% of cases were squamous cell carcinoma, 22.9% were adenoid cystic carcinoma), nasal obstruction was found in 95.7%, cheek swelling in 98.6%, facial asymmetry in 98.6%, palpable mass in the upper buccal sulcus 94.3%, epistaxis 80%, loss of smell 52.9%, exophthalmos 50%, oroantral fistula 55.7%, numbness in the cheek and infraorbital skin 58.6%, palpable lymph node in the neck 35.7%, diplopia 34.3%. The most common early clinical signs and symptoms were nasal congestion, followed by anosmia, paresthesia, epistaxis and numbness. The most common late clinical signs and symptoms included palpable buccal mass, followed by paresthesia, lymphadenopathy and diplopia [11]. All 3 of our patients complained of nasal obstruction and one patient with lymphoma complained of cheek swelling. In the study of Devajara et al. involving patients with sinonasal malignancy, the most common histopathologic finding was squamous cell carcinoma followed by adenoid cystic carcinoma. Stage 4 (42/58) disease was detected in most of the cases. The most common location of the tumor was in the maxillary sinus (55.7%), followed by ethmoid sinuses and nasal cavity. In the study, epithelial histopathology was statistically associated with poor prognosis [10]. Arosia et al. found that maxillary sinus floor involvement was a negative prognostic factor on survival rate and local control rate [2]. Even in early stage tumors, locoregional control and survival rates are disappointing. The reasons for this, besides the deficiencies in the staging system, may be that early stage maxillary cancers involve critical areas and cannot be treated with minimally invasive endoscopic endonasal approaches [2]. Contrast-enhanced CT and MR imaging are used in the diagnosis. Endoscopic endonasal examination is performed and biopsies are taken [2]. A Caldwell-Luc approach may be required to obtain biopsies from non-mucosal lesions in the nasal cavity or gingivobuccal region that do not form a significant mass. In our cases of lymphoma and adenoid cystic carcinoma, biopsy was planned with the Caldwell-Luc approach. In the case of pleomorphic adenoma ex carcinoma in our study, preoperative punch biopsy of the mass in the nasal cavity was first thought to be sinonasal papilloma, and the second biopsy was reported to be compatible with pleomorphic adenoma. The presence of a large, diffuse and destructive lesion in this case should always suggest the possibility of ex-carcinoma in addition to pleomorphic adenoma. After the diagnosis of cancer, spread to other sites is investigated with contrast-enhanced whole-body CT or PET. Although mucosal and bone involvement can be evaluated with preoperative imaging, intraoperative endoscopic examination and frozen examination may show different rates of involvement. In surgical treatment, total maxillectomy, lateral rhinotomy, partial maxillectomy, craniofacial resection and extended maxillectomy as well as combined approaches including endoscopic endonasal approaches can be applied [2,10]. However, complete resection is not always possible due to complex anatomy and proximity to vital and functional structures [9]. In addition to surgical treatment, chemotherapy and radiotherapy can be applied. In poorly differentiated tumors, induction chemotherapy may be given. Adjuvant radiotherapy may be given in locally advanced tumors and in the presence of lymphovascular and/or perineural invasion. In cases with positive surgical margins, chemotherapy and postoperative concomitant chemoradiotherapy may be given [2]. Palatomaxillary resection and extended surgery may require the use of flaps or obturator prosthesis for reconstruction [2,4,8]. In the study by Arosia et al, the preoperative N positivity rate in patients with palatomaxillary cancer was as low as 2.6% (N1). 31 patients (26.2%) had positive surgical margins. Adjuvant treatment was required in 71.2% of patients (RT in 66.4%, CRT in 6.8%). Total recurrence rate was 40.7% [2]. Pleomorphic adenoma ex carcinoma accounts for 2-4% of salivary gland tumors arising from pleomorphic adenoma [3]. Pleomorphic adenoma ex carcinoma of the maxillary sinus is extremely rare [3,12]. Rapid growth or recurrence of a long-standing pleomorphic adenoma should suggest malignant change. Recurrences and metastases are common in pleomorphic adenoma ex carcinoma. Survival is poor in metastatic disease [3]. CT may show a large solid mass with focal areas of necrosis. There may be bone destruction, nasal cavity, maxillary sinus and hard palate extension [4,5]. Surgical treatment requires approaches including endoscopic sinus surgery and maxillectomy procedures depending on the extent [3,4,12]. Postoperative radiotherapy improves local control rates and has a positive effect on survival. Radiotherapy is indicated for perineural invasion, positive margin, high grade histology, neck metastases and recurrence. Chemotherapy is indicated in postoperative recurrences, inoperable and metastatic diseases [3]. Recurrences may require more than one surgery [12]. In the case of pleomorphic adenoma ex carcinoma in our study, the tumor was removed macroscopically in the second surgery and the gingival arch and soft palate were left in place so that the patient could use an obturator prosthesis. Ex carcinoma was reported as high-grade adenocarcinoma with lymphovascular invasion. Adjuvant chemoradiotherapy was administered due to microscopically positive surgical margins. Adenoid cystic carcinomas are rare malignant tumors of the major and minor salivary glands. Involvement of the nasal cavity and paranasal sinuses is rare [7]. Adenoid cystic carcinomas are slow-growing malignancies with a high local recurrence rate and are capable of distant metastasis (lung and bone) [7,9]. In the study of Michel et al. 72% of 25 cases were diagnosed at locally advanced stages (T3 and T4). In order of frequency, they were localized in the maxillary sinus, nasal cavity and ethmoid sinuses. Surgical treatment includes maxillectomy procedures, different degrees of ethmoid sinus resections and skull base procedures when necessary [7]. Obturator prosthesis is used for surgical palate defects [4]. In the study of Michel et al., surgical margin positivity was found in 65% of the operated cases [7]. In a meta-analysis of adenoid cystic carcinomas of the paranasal sinuses and nasal cavity, the most common site was the maxillary sinus (63%, 63/99). Most of the cases were in advanced stage when detected (stages 3 and 4). In 81% of 99 cases, invasion of surrounding tissues (orbit, dura, cavernous sinus, brain, muscles and skin) was detected. In addition, 51% perineural invasion and 43% positive surgical margins or close surgical margins were observed [13]. In the case of adenoid cystic carcinoma in our study, intraoperative frozen examination was verbally interpreted as benign. After the definitive diagnosis of malignancy, the patient was offered maxillectomy, but since the patient refused surgical treatment, he was referred to radiochemotherapy. In a review study of 774 cases of head and neck adenoid cystic carcinomas other than major salivary glands, neck metastasis was found in 5.3%. Elective neck dissection may not be performed due to low neck metastasis [14]. Sinonasal lymphomas are extremely rare and may mimic benign processes. In one study, extranodal NHL of the sinonasal tract was found in 1.63% of 1457 patients with NHL [15]. It may present as an isolated lesion or with systemic disease symptoms. Histopathologically diffuse large B-cell lymphoma is more common in the sinonasal region. Extranodal natural killer/T cell lymphoma was observed in the second frequency [6,15,16]. The use of CT, clinical suspicion and biopsy play a key role in the diagnosis [15]. Lymphomas may mimic unilateral chronic rhinosinusitis; therefore, tissues obtained during endoscopic sinus surgery should be sent for histopathologic examination [6]. In our case, there was a mucosal thickening appearance on CT (Figure-1) which could be confused with chronic rhinosinusitis. However, the presence of bone destruction with mucosal thickening should raise suspicion for malignancy. Symptoms such as nasal obstruction, intranasal mass, unilateral epistaxis, facial swelling, palatal lesion, and visual differences may be observed [15]. Extranodal NK/T-cell lymphoma- nasal type may cause ulcerative destructive lesions on midline facial structures [16]. Unlike SCC, NHL is usually submucosal and has a large appearance. SCC is usually ulcerative [15]. However, intranasal lesions may not be differentiated from other pathologies. Biopsy is necessary for this [15]. CT may show mass, bone destruction and skull base involvement [6,15]. In a study conducted to differentiate non-hodgkin lymphoma and squamous cell carcinoma located in the maxillary sinus by imaging methods, there was no significant difference in MR signal intensities and SUVmax values, while intratumoral necrosis on CT was found statistically significantly more frequently in squamous cell carcinoma. In non-hodkin lymphomas, the presence of the maxillary sinus wall between the tumor was found to be significant in relation with the tumor growth pattern on CT [17]. The CT finding of our lymphoma case supports this. The CT scan of our case (Figure-1) shows a defect in the maxillary sinus wall with a lesion intervening the intact wall from inside and outside. AIDS is positive in 4-10% incidence of NHLs. AIDS is rare in paranasal sinus NHLs [15]. T cell lymphomas are much rarer in the head and neck region. Chemotherapy and radiotherapy constitute the main treatment for lymphomas [6]. ConclusionIn conclusion, rare malignancies other than squamous cell carcinoma may be encountered in the maxillary region. Non-hodgkin lymphomas in this region may mimic rhinosinusitis. The presence of bone destruction on CT is important in differentiation from rhinosinusitis. Maxillary pleomorphic adenoma ex carcinoma caused a large destructive mass affecting the entire palatomaxillary region in our case. Preoperative biopsies of these cases may be reported as pleomorphic adenoma because they were taken from a part of the tumor. In large and destructive lesions, the possibility of ex-carcinoma should be considered. Maxillary adenoid cystic carcinomas may present as locally destructive lesions as in our case. In the diagnosis and treatment of these lesions, open surgical approaches should be combined with endonasal endoscopic approaches. References
Presented atOlgulardan biri olan Pleomorfik adenoma ex karsinoma olgusu elektronik poster olarak Türk Rinoloji Kongresinde sunulmuştur (Turkish Rhinology Congress in 2023, May 17-20, EP-06, İzmir, Türkiye). |
|||||||||||
| Keywords : paranazal sinüs neoplazmları , maksilla , lenfoma , karsinoma | |||||||||||
|