|
|||||||||
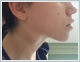
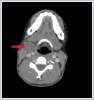
AbstractTularemia is a zoonotic infection caused by Francisella tularensis, which is also known as rabbit fever, fowl fever, tick fever, O'Hara’s disease, and Francis’ disease. This disease is transmitted by arthropod bites, inhaling contaminated aerosols, or drinking contaminated water. There are several forms of tularemia, including the ulceroglandular, oropharyngeal, oculoglandular, typhoidal, and pneumonic forms. Oropharyngeal tularemia patients are often misdiagnosed as having upper respiratory tract infections. In this article, we have reported two rare cases of oropharyngeal tularemia with tonsillitis and lymphadenitis in the neck that were unresponsive to penicillin treatment.IntroductionTularemia is a zoonotic infection that is commonly seen in Turkey and around the world. It is caused by Francisella tularensis bacteria, and is also known as rabbit fever, fowl fever, tick fever, O'Hara’s disease, and Francis’ disease. This bacteria is a small, gram negative, aerobic, and immobile coccobacillus that requires an enriched media for reproduction, and it can replicate in cells. F. tularensis is divided into three subtypes, tularensis (type A biovar), holarctica (type B biovar), and mediasiatica, and all of these cause human infections [1]. Rabbits, mice, squirrels, and other rodents are the major reserves for this disease. Humans become infected via arthropod bites, inhaling contaminated aerosols, and drinking contaminated water [2]. Despite this organism’s durability in damp and cold outdoor environments, high temperature, direct sunlight, and water chlorination can shorten its life span [1]. Depending on the type of bacteria and the site of entry into the body, five clinical forms can be described: ulceroglandular, oculoglandular, oropharyngeal, typhoidal, and pneumonic [3]. While ulcerative tularemia is the most common clinical form in North America, oropharyngeal tularemia cases are seen more frequently in Turkey and Europe [4]. In oropharyngeal tularemia, direct bacterial invasion of the oropharynx is seen, with an incubation period varying between 1 and 14 days [3,4]. Infections are often seen in more than one person from the same family at the same time. Unfortunately, oropharyngeal tularemia patients are often misdiagnosed as having upper respiratory tract infections and treated with beta lactam antibiotics, but they do not get better [4]. In this article, we presented two cases of oropharyngeal tularemia from the same region in 2016 who presented to our clinic with tonsillitis.Case ReportCase Report 1 A 64-years-old female patient presented to our clinic with complaints of a sore throat, weakness, fever, and generalized muscle pain over the previous two weeks. Amoxicillin was prescribed by the general practitioner and clindamycin was prescribed by the otorhinolaryngologist, but the patient’s symptoms persisted. This patient stated that she lives in a village and is involved in animal husbandry. Upon physical examination, the right tonsil was hypertrophied and exudative, but the left tonsil was normal. On the right side of the neck, in the jugulodigastric region, a 3x2 cm area of hard semi-mobile precise lymph nodes was palpated (Figure 1)
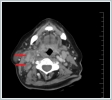
The other physical examination findings were normal. This patient’s temperature was 38.2ºC. The hemogram results were as follows: leukocytes 16,500/ml, hemoglobin 12 g/dl, platelets 342,000/μl, erythrocyte sedimentation rate (ESR) 79 mm/h, and C-reactive protein (CRP) 17 mg/l. The contrast-enhanced cervical computed tomography (CT) of the patient showed that at bilateral levels 2, 3, and 5 (the largest was 23x21 mm on the right side at level 2), multiple lymphadenopathies compatible with cystic-necrotic degeneration in the center were observed (Figure 2).
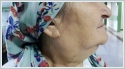
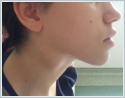
A Tru-Cut biopsy was performed on the right lymphadenopathy and reported as granulomatous lymphadenitis. The microagglutination test (MAT) for F. tularensis was positive (a fourfold or greater increase in the antibody titre was accepted as diagnostic in the recurring tests performed two weeks later). Doxycycline was given orally to the patient twice a day for three weeks. At the follow-up during the 4[th] week, suppuration was observed at the lymphadenopathy on the right side of the neck. Therefore, a consultation was requested from the infectious diseases clinic. Streptomycin was begun at a dosage of 1 g twice a day (intramuscularly) for 14 days. The suppuration continued for 10 days after treatment. The areas were dressed daily and healed uneventfully. In addition, pure tone audiometry was performed before and after the medical treatment, with no change detected. This patient has reached her 7[th] month follow-up without any complaints. Case Report 2 An 18-year-old female patient presented with complaints of a sore throat, fever, and weakness for 1 week. The patient stated that she used oral amoxicillin + clavulanic acid for 5 days; however, her symptoms continued. She also stated that she had neighbors who recently came to our clinic with similar complaints. Upon physical examination, her right tonsil was hypertrophied and exudative, but the left tonsil was normal. A 4x4 cm semi-mobile sensitive lymphadenopathy was observed in the jugulodigastric region on the right side of her neck (Figure 3)
The other physical examination findings were normal. This patient’s temperature was 37.9ºC. The hemogram results were as follows: leukocytes 8,100/ml, hemoglobin 13.6 g/dl, platelets 247,000/μl, ESR 20 mm/h, and CRP 0.5 mg/l. The contrast-enhanced cervical CT showed a well-defined pathological lymph node with a heterogeneous internal structure and peripheral and septal contrast-enhanced necrotic areas (Figure 4).
Because oropharyngeal tularemia was suspected, a microagglutination test was ordered. The results of the test were positive (1/640) so a consultation from the infectious diseases clinic was requested. Streptomycin at a dosage of 1 g twice a day (intramuscularly) for 14 days was recommended. Pure tone audiometry was performed before and after the medical treatment, with normal results. At the end of the first month, the cervical lymph nodes were completely healed. This patient has reached her 7[th] month follow-up without any complaints. The dimensions were 25x20x48 mm as observed in the right anterior cervical chain at level 2. The cervical magnetic resonance imaging (MRI) on the right side of the neck at level 2 showed conglomerate lymph nodes (26x20x40 mm) with cystic-necrotic centers and heterogeneous internal structures. DiscussionThe infective dose of F. tularensis varies, depending on the type of entry and type of microorganism. It is believed that the bacterium grows in the area where it enters, reaches the regional lymph nodes, and then spreads via the lymphohematogenous route to many organs. The oropharyngeal form develops via the bacterial contamination of the oral mucosa during the intake of water and food. The initial symptoms often mimic the common cold, with the most common symptoms being myalgia, arthralgia, weakness, loss of appetite, weight loss, headache, and fever [5]. The patients who presented at our clinic exhibited a similar clinical picture and were treated with the diagnosis of acute tonsillitis. However, they showed no response to that treatment. Preauricular, submandibular, and anterior cervical lymphadenopathy are seen in the vast majority of oropharyngeal tularemia patients. In Atmaca's case series, it was stated that the most frequently observed region was Level 2 [6]. The lymphadenomegaly is usually unilateral, not painful to any significant degree, moderately hard, does not adhere to the surrounding tissues, mobile, and swirling and sweeping in the late period [7]. In both cases presented here, there were nonpainful lymph nodes on the right side of the neck at level 2, in accordance with the literature. Unfortunately, routine laboratory tests and radiological imaging methods are nonspecific in tularemia cases. Moreover, the white blood cell values and ESR may be normal or elevated in the laboratory tests [7]. In our first case, the ESR, CRP, and leukocyte levels were high; however, all of the values were in the normal range in our second case. The Rose Bengal test, Brucella Coombs gel test, sputum acid and alcohol resistant bacteria (AARB) staining, and purified protein derivative (PPD) test were negative in both cases. The throat swab culture test showed normal bacterial flora. In the radiological findings, the neck CT showed enlarged lymph nodes, with or without central necrosis, and the peripheral enhancement showed the formation of unilocular or multilocular abscesses. Radiologically, the cervical CT showed enlarged lymph nodes, with or without central necrosis, and unilocular or multiloculated abscess formation with peripheral enhancement [7]. The radiological findings of our patients (multiloculated peripherally-enhanced lymph nodes compatible with central cystic-necrotic degeneration) made us suspect tularemia. In cases of tularemia, serological tests are used most frequently in the diagnosis due to the difficulties with culture and isolation, and to avoid the risk of hand contact. The MAT is the test most frequently used for this purpose [4], and it is positive about 10–14 days after the onset of the disease [8]. In the MAT, an antibody titre greater than 1/128, or a fourfold or more increase in the antibody titre in recurring tests with a two-week interval, is considered to be diagnostic [7]. In our cases, the MAT was used in the diagnosis. In our first case, the antibody titre increased fourfold in the repeated tests, while in the second case, the antibody titer was 1/640. Other than the MAT, a hemagglutination assay, enzyme-linked immunosorbent assay (ELISA), and western blot can also be used to detect antibodies against F. tularensis. However, the fastest and most sensitive method is to use a polymerase chain reaction (PCR) assay. A PCR analysis can be performed without any specific fixation on the specimen obtained from the suspected lymph node [7]. A histopathological examination of a tularemic lymph node reveals central necrosis, with a chronic granulomatous type of inflammation around the lesion, surrounded by epithelioid histiocytes (macrophages), lymphocytes, and giant cells [9]. The detection of granulomatous inflammation in tularemia requires a differential diagnosis with infectious and noninfectious diseases. The noninfectious causes include sarcoidosis, a foreign body reaction, collagenosis, and other granulomatous diseases of unknown origin. The infectious causes include bacterial diseases (such as tuberculosis, brucellosis, yersiniosis, salmonellosis, and cat scratch disease), parasitic diseases (such as toxoplasmosis and leishmaniosis), and fungal diseases (such as sporotrichosis and candidiasis) [1]. Approximately one-half of the patients develop suppurative inflammation and 8% develop caseous necrosis, which may be interpreted as tuberculosis lymphadenitis and lead to erroneous treatment approaches [5]. Even though there is quite similarity in terms of imaging and cytopathologic findings between tuberculosis and tularemia, tuberculous adenitis is usually seen in the bilateral supraclavicular region and posterior triangle of the neck, whereas tularemia causes usually unilateral lymphadenopathies at level 2 and 3 [10]. Although the bactericidal effects of aminoglycosides and low relapse rate are advantageous in the treatment of tularemia, the obligatory parenteral administration of these antibiotics and ototoxicity are disadvantages. Chloramphenicol and tetracycline are bacteriostatic and pose a high risk for relapse; however, doxycycline may be preferred in many cases of type B F. tularensis. Bacteriostatic drugs should be chosen over the first 2–3 weeks of onset in order to minimize the relapse risk. Quinolones are highly effective against F. tularensis and are currently used in therapy [11]. The treatment duration is 10–14 days for the bactericidal drugs and 14–21 days for the bacteriostatic drugs, and treatment success is defined as the disappearance of the affected lymph nodes along with the signs and symptoms. Moreover, the initiation of treatment during the first 3 weeks of the disease has been reported to increase its success [12]. In general, small lymphadenopathies are regressed with streptomycin therapy, while surgery is inevitable for large solitary lymph nodes and especially in the presence of a fluctuant abscess [13]. The principle of disease prevention is to avoid contact with the bacteria. Masks and gloves are required for laboratory work, water resources should be protected, and frequent control are important in protecting against this disease [11]. In addition, gloves, masks, and goggles should be used when coming in contact with hunting animals. It is recommended that doxycycline or ciprofloxacin is given for 14 days to those persons who may have come in contact with F. tularensis as prophylactic therapy. Unfortunately, immunoprophylaxis is not yet available [4]. In conclution, otolaryngologists should not overlook oropharyngeal tularemia in patients with acute tonsillitis in endemic areas, or in patients with cervical lymphadenitis who have not responded to penicillin treatment. References
|
|||||||||
| Keywords : Tularemi , tonsillit , lenfadenit | |||||||||
|