|
|||||||
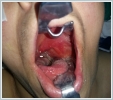
AbstractGlomus vagale is a rare variant of head and neck paragangliomas arising from neuroendocrine cells. Although these tumors are highly variable in their location, size, and functionality, they are mostly detected as a mass in the neck. Treatment depends on the general condition of the patient, the location of the disease, and the symptoms it causes. We reported the case of a 71-year-old female who was admitted to our clinic because of unexpected respiratory distress of approximately 1-month duration.IntroductionParagangliomas are rare neuroendocrine tumors arising from chromaffin-negative cells of the neural crest. They constitute 0.6% of all head and neck tumors and 95% are nonfunctional. The carotid body, vagus ganglion, jugular bulb, and tympanic plexus are typical anatomic sites of paragangliomas [1]. Glomus vagale is a paraganglioma originating from the lower, middle, or upper ganglia along the vagus nerve, and they account for 5% of all head and neck paragangliomas [2]. Paragangliomas, mostly bening non-functional tumors, are presented to clinicians with symptoms and/or findings caused by some factors such as the location, the size and the pressure effect. While a long standing neck mass without pain may be a finding of glomus caroticum, pulsatile tinnitus and/or conductive hearing loss may be prominent findings in tumors including glomus jugulare and glomus tympanicum. A vagal tumor located between the carotid bifurcation and the jugular foramen may invade the carotid artery or cause cranial nerve disorders or skull-base destruction [2]. In this article, we presented a patient with glomus vagale placed in the left parapharyngeal space. This case stood out with unique presentation owing to respiratory distress revealed by a vagal tumor. Case ReportA 71-years-old female was admitted to our clinic with respiratory distress of 1-month duration. A painless, non-motile mass approximately 3×3 cm in size, located in the left submandibular area and reaching under the left auricula, was detected. The mass pushed the left tonsil bed to the right side of the median line in the mouth and thus narrowed the oropharynx (Figure 1).
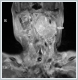
Patient’s only symptom was respiratory distress. Her vocal cord movements and the cranial nerve examination were normal. There was also no evidence of Horner’s syndrome. On the first day of admission, the patient suffered increased respiratory distress and therefore underwent tracheotomy under local anesthesia. Evaluation of 7-year-old neck magnetic resonance (MR) images revealed a left parapharyngeal mass 55 ×35 mmin size that narrowed the pharyngeal airway. Its imaging features consisted of a hypo-isointense lesion on T1-weighted images, a non-homogenous hyperintense lesion with ‘salt-pepper’ appearance on T2-weighted images, and strong contrast enhancement (Figure 2).
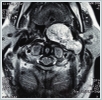
Computed tomography (CT) of the neck, magnetic resonance imaging (MRI), and MR angiography of the neck were performed. While skull-base erosion was not detected on the neck CT, the neck MRI showed a lesion filling the left parapharyngeal space that was hypo-isointense on the T1-weighted and hyperintense on the T2-weighted images. The mass also showed intense gadolinium uptake on the T1-weighted images, presenting as a sharp-smooth, confined, solid mass, 63 × 41 ×65 mm in size and narrowing the nasopharynx-oropharynx-hypopharynx air column (Figure 3). Doppler ultrasound showed no decrease in either common carotid or internal-external carotid artery (I-ECA) flow velocities compared to the unaffected side.
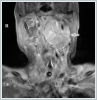
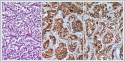
The patient was administered general anesthesia and then underwent mass excision via a transcervical-transparotidapproach. Flap elevation revealed a submandibular mass that displaced the I-ECA anteriorly and the internal jugular vein (IJV) posteriorly. The 12[th], 11[th], and 7[th] cranial nerves were identified but the enormous size of the mass prevented a determination of its origin (lower, middle, or upper vagal ganglion). The pathology report on the completely excised mass was paraganglioma. Microscopically, the tumor exhibited a Zellballen pattern of cuboidal cells with organoid sequences surrounded by fibrovascular septa (Figure 4). Immunohistochemically, intense synaptophysin and chromogranin positivity were observed (Figure 4B, 4C). In the first post-operative week, the patient’s tracheotomy was closed. Her left vocal cord was paralytic but at the end of the eighth postoperative month she was without respiratory distress.
DiscussionVagal paragangliomas have been rarely reported in the literature. Given the slow growth pattern of these tumors, patients require close follow-up because of the potential involvement of the cranial nerves, main vasculature, and skull base. Paragangliomas are mostly sporadic and occur in older individuals (50–60 years of age). The majority of clinicians prefer a conservative approach to treatment based on clinical experience and the low malignancy rate of paragangliomas [3]. The 71-year-old female patient reported on by Wang et al. [4] underwent MRI follow-up eight times over 7.4 years. The growth rate of her axially spreading tumor was 0.68 mm per year, compared to 0.66 mm per year for the tumor in our patient. Vagal tumors are separated into three types according to their relation to the skull base. Type A tumors are limited to the cervical region; type B abuts the jugular foramen and skull base with either anterior displacement and/or encasement of the ICA; and type C extend into the jugular foramen, often with intracranial extension [2]. Our patient had a type B tumor, with posterior displacement of the IJV and anterior displacement of the I-ECA. In a review of 46 cases of glomus tumors reported in a 20-year period, 46% consisted of only a mass, 39% were associated with tinnitus, and 39% of the masses were pharyngeal [2]. None of the respective patients presented with respiratory distress whereas in our patient the parapharyngeal mass was easily visible on inspection and induced respiratory distress. Ovoid head and neck paragangliomas can be detected using CT and MRI. CT also allows the evaluation of skull base erosion whereas on MRI signal enhancement on the T2-weighted scans and a strong enhancement of the mass on the T1-weighted images are seen after gadolinium administration. T1-weighted imaging also reveals a mass that is iso-hypointense compared to muscle and, when larger, has a ‘salt-and-pepper’ appearance on the T2-weighted scans. Punctate regions of hemorrhage or slow flow cause the ‘salt’ appearance, and flow voids the ‘pepper’ appearance [5]. The distinction of tumors including glomus vagale, carotid body tumor, sympathetic tumor can be made based on imaging studies (contrast-enhanced CT, MRI, angiography), relatively. A vagal tumor pushes I-ECA anteromedially whereas lateral displacement is typical in sympathetic tumors, and ‘lyre sing’ is an important finding for carotid body tumor that is obtained by angiography [6]. Angiography and accompanying embolization are not considered appropriate by some surgeons [6] whereas Browne et al. [7] emphasized the need for preoperative angiography and embolization in some tumors (particularly type C) to reduce bleeding during surgery. In patients with vessel invasion, carotid balloon occlusion tests should be performed to assess the status of collateral vessels in the brain in case of a possible intraoperative vessel resection [8]. However, preoperative angiography (with/without embolization) was not performed in our patient, and anterior displacement of I-ECA was taken attention in MR angiography. Paragangliomas with local-distant metastases and rapid enlargement are considered malignant but their histopathological differentiation from benign lesions is not possible [9]. The malignancy rate of glomus caroticum is 5–10% and that of vagal tumors 16%. Surgical approaches are not recommended for vagal tumors in the absence of vagus paralysis and pressure effects on surrounding tissues, including skull base invasion. If a vagal tumor is encountered intraoperatively in a patient diagnosed preoperatively with glomus caroticum, surgery should be canceled and the patient followed up or treated with radiotherapy [10]. In patients with paraganglioma, the treatment options are surgical excision, radiotherapy, embolization, and observation. If there is a high probability of glomus vagale based on the results of imaging studies, if the tumor is a non-functional, slow-growing mass that does not cause cranial nerve deficit, and if there are no findings related to mass pressure, then follow-up is, in our opinion, the best approach. However, surgery is inevitable in a patient who has intolerable symptoms due to pressure on the surrounding tissues, as was the case in our patient. References
|
|||||||
| Keywords : Glomus vagale , Paraganglioma , Solunum sıkıntısı | |||||||
|