|
|||
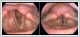
AbstractThe coronavirus-19 (Covid-19) disease, which started in December-2019 and has affected the whole world until today, has become a pandemic that causes some neurological complications. In order to prevent the epidemic, the mRNA vaccines produced were approved by the food & drug administration (FDA) in December-2020 and vaccination campaigns were started. I present a case of unilateral vocal cord paralysis(VCP) after the first dose of Covid-19 mRNA vaccine in a 61-year-old male patient with no history of systemic disease.IntroductionCovid-19, caused by acute respiratory syndrome coronavirus-2 (SARS-CoV -2), started in Wuhan province of China in December-2019 and became an epidemic worldwide within a few weeks. Covid- 19 is a viral infection that causes symptoms such as myalgia, cough, runny nose, sore throat, shortness of breath, and loss of taste and smell. It can cause a wide range of problems such as Acute Respiratory Distress Syndrome, Multi-organ failure and Neurological problems [1]. It is very important to pay attention to sanitation, as well as the use of masks and vaccination in the prevention of infection. FDA approved two vaccines for the prevention of COVID-19 infection in December 2020. Numerous adverse events have been reported in clinical trials of the Pfizer- BioNTech vaccine, ranging from mild symptoms including but not limited to injection site pain, myalgia, fatigue and fever to more serious side effects including anaphylactic shock [2,3]. However, as far as we know, VCP after the fisrt dose of Covid-19 Pfizer- BioNTech vaccination has not been reported in the literature. I present a case report of hoarseness after the first dose of the vaccine. Case ReportA 61-year-old male patient without a significant medical history was applied to the ENT outpatient clinic with sudden hoarseness. The first dose of the Covid-19 mRNA vaccine (Pfizer- BioNTech) the vaccine was administered approximately 24 hours before the onset of hoarseness in the anamnesis. There were no complaints such as difficulty swallowing, shortness of breath or palpitations. In the flexible laryngoscopic examination of the patient, the left vocal cord was observed to be paralytic in the paramedian position. Rima glottis was approximately 5-6 mm during inspiration and 2-3 mm during phonation. Piriform sinus and other laryngeal structures were observed naturally (Video 1).
The patient had Gag reflex. Uvula and tongue were in the midline and no palatoglossal and pharyngeal sensation defects were observed. The patient's vital signs were stable. Contrast-enhanced Neck and Thorax Computed Tomography (CT) and Cranial Magnetic Resonance (MR) imaging were performed for the etiological evaluation. In addition, extensive biochemical tests, including complete blood count, thyroid, kidney and liver function tests, lipid profile, serology, serum electrolytes and complete urinalysis were performed. There was not pathology detected in imaging and laboratory examinations. It was concluded that mRNA vaccine was the cause of VCP. Metilprednisolon treatment was started at 1 mg/kg and reduced other every day for the patient. The second dose of the mRNA vaccine was administered 28 days after the first dose of vaccine. Voice therapy was recommended to the patient who did not have any problems after the second dose of vaccine administration, but the patient did not accept it. No changes were detected in vocal cord paralysis during 6 -month follow - up. Verbal informed consent was taken from the patient. DiscussionVocal cord paralysis refers to decreased and missing movements of the vocal cords. It can be multifactorial and is a very common sympytom of underlying diseases. Clinical signs occur due to damage to the vagus nerve, superior laryngeal or recurrent laryngeal nerves. Patients have symptoms such as hoarseness, cough, aspiration, shortness of breath, and dysphagia [4]. Thyroid, larynx, esophagus, lung tumors, thyroidectomy, esophageal and cardiac surgical procedures are frequently detected factors in VCP etiology. The most common situation in clinical practice is idiopathic vocal cord paralysis [5]. In our article, the development of vocal cord paralysis in which no etiological cause could be found, after mRNA vaccination, is remarkable. Due to the increased morbidity and mortality from Covid-19, collective vaccination programs have been applied all over the world. It is essential to understand the epidemiology of the disease and the adverse effects of vaccination. Insufficient understanding of these issues can lead to extra morbidity and mortality [6]. Thanks to the COVID-19 mRNA vaccines approved by the FDA, it begins to identify the spike protein found on the surface of SARS-COV-2, the virus that causes Covid-19 in the human body. The immune system recognizes the spike protein as an invader and produces antibodies against it. Antibodies formed thanks to the vaccine, after the virus enters the body, they recognize and neutralize the virus before it causes disease [7]. The immune response that develops after vaccination may be a trigger for the onset of autoimmune diseases in some people. This mechanism is held responsible especially for the neurological problems such as Guillain-Barré syndrome, Bell's palsy, trigeminal neuralgia or glossopharyngeal neuralgia that developed after vaccination. There are advanced neurological complications after the first dose of Covid-19 mRNA vaccine in the literature [8,9]. There are some articles of vocal cord paralysis developing after Covid -19 mRNA vaccine in the literature[10,11]. However, to the best of our knowledge, our submission is the first publication in Turkey to report the development of VCP after mRNA vaccination. . ConclusionA case of vocal cord paralysis after the Covid-19 mRNA vaccine is reported in this study. It is critical for clinicians to quickly recognize the development of neurological or systemic complications after vaccination. It should be emphasized that the Covid-19 mRNA vaccine has a low adverse effect profile and that the benefit of the vaccine outweighs the potential side-effect problems, both on an individual and societal basis. We encourage and support the recommendations of the CDC and WHO guidelines for the COVID-19 vaccine. References
|
|||
| Keywords : Vokal kord paralizisi , Covid-19 , mRNA aşı | |||
|