|
|||||||||||||
AbstractAmiloidosis is a diasese characterized by accumilation of amyloid protein on several organs of the body. Although systemic involvement is more common, localized laryngeal involvement quite rare. In this case, a 59-year-old male patient presenting with hoarseness to our clinic was discussed. After laryngoscopic examination, multiple biopsies were taken from irregular masses were observed on anterior commissure of vocal cord, the petiole of epyglottis and subglottic area. Histopatological findings were consistent with amyloid deposition, incluiding Congo Red and crystal violet positivity. Whilst localized amyloidosis is rare, it should be considered as a differential diagnosis of larygngeal masses.IntroductionAmyloidosis is a group of diseases characterized by the deposition of abnormal proteins (insoluble fibrillar proteins) in the extracellular space of the body. The accumulation can be systemic or localized [1]. While amyloid accumulation is possible in several organs of the body (heart, liver, kidney, spleen, etc.), laryngeal involvement is exceedingly rare [2]. Localized laryngeal amyloidosis accounts for 0.2–1.2% of all benign laryngeal tumors. Nevertheless, the larynx remains one of the most commonly affected organs in the neck region. It is more common in males than in females and peaks in the fourth to sixth decades of life [3]. Although symptoms of respiratory amyloidosis are nonspecific, they may include cough, dyspnea, wheezing, hemoptysis, and dysphonia [4]. In this case, a patient presenting with dysphonia was evaluated. Furthermore, the data extracted from the reviewed articles including age, gender, symptoms, laryngeal localization of amyloidosis, treatment strategies, and follow-up durations are discussed in conjunction with the present case and summarized in Table 1.
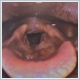
Case ReportA 59 year old male patient presented to our clinic with a complaint of hoarseness that had persisted for 4 months, without any additional symptoms such as dyspnea, cough, or dysphagia. The patient had no known comorbidities or family history and had a history of 30 pack-years of smoking. On physical examination, otoscopy, rhinoscopy, and intraoral examination appeared normal, while laryngoscopic examination revealed irregular masses on the anterior commissure of the vocal cords, the petiole of the epiglottis, and the subglottic area (Figure 1), which led to the decision for a biopsy.
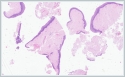
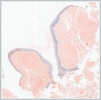
Histopathological analysis of the multiple biopsies revealed amorphous material compatible with amyloid in the subepithelial area, confirmed by positive Congo red and crystal violet staining (Figure 2,3).
To differentiate between systemic and localized amyloidosis, further investigations were requested through an internal medicine consultation. The patient's laboratory results, including complete blood count, liver and kidney function tests, serum calcium, potassium, erythrocyte sedimentation rate, and serum immunoglobulins, were within normal limits. Protein levels in serum and urine were assessed, and both were found to be normal. No monoclonal proteins were detected in serum and urine protein electrophoresis. The electrocardiogram and chest X-ray were normal. After excluding diseases that could cause systemic amyloidosis, it was determined that the amyloidosis was localized to the larynx. Given the improvement in the patient’s symptoms, close follow-up and continued monitoring were deemed appropriate for the treatment plan. Follow-up evaluations were performed at the first, third, and sixth months, during which endoscopic examinations demonstrated no signs of lesion progression or the emergence of new formations. DiscussionAmyloidosis is a disease characterized by the accumulation of insoluble abnormal proteins in the extracellular space, resulting in dysfunction across various organs. This condition, which may be either systemic or localized, is relatively rare in the larynx [5]. Although its etiology is not fully understood, several theories propose that plasma cells may produce amyloid light chains, or that there may be deficiencies in the degradation of these light chains within the body [6]. In certain cases, genetic mutations have also been identified as a contributing factor [7]. Depending on whether the condition is systemic or localized, the type of amyloid protein involved may vary. The most commonly encountered forms include AL (amyloid derived from immunoglobulin light chains; the most frequent type), AA (amyloid derived from serum amyloid A protein), ATTR (amyloid derived from the transport protein transthyretin), and dialysis-related amyloidosis (amyloid composed of beta-2 microglobulin) [8]. In systemic amyloidosis, amyloid deposits accumulate in organs such as the liver, heart, spleen, and kidneys, leading to clinical manifestations including hepatomegaly, cardiomegaly, proteinuria, and macroglossia [8]. Localized laryngeal amyloidosis accounts for 0.2–1.2% of all benign laryngeal tumors. However, the larynx is the most commonly affected organ in the head and neck region in cases of amyloidosis [1]. A review of eleven laryngeal amyloidosis cases published in the last five years showed that most of the patients had amyloid deposits localized to the glottis (Table 1). In our case, deposits were observed not only in the glottis but also in the supraglottic and subglottic regions. The mean age of the patients was 38 years, ranging from 14 to 73 years. The study included five male and six female patients [1,9–13]. In laryngeal amyloidosis, the most common symptom is hoarseness, although some patients may present with dyspnea, stridor, persistent dry cough, sleep apnea syndrome, or dysphagia [1]. A review of recent cases demonstrated that the primary symptom of laryngeal amyloidosis was dysphonia, with stridor and dyspnea also reported as accompanying findings [1,9–13,14]. Our patient likewise presented to our clinic with dysphonia. On laryngoscopic examination, amyloid deposition may appear in various forms, including nodular, polypoid, or diffuse infiltration. The differential diagnosis should include neoplastic diseases, fibroma, papilloma, and tuberculosis. The gold standard for diagnosis is histopathological analysis, in which amyloid deposits are stained with Congo red and exhibit apple-green birefringence under polarized light microscopy [5]. Following the diagnosis, further investigations including complete blood count, serum calcium, sodium, and potassium levels, liver and kidney function tests, serum immunoglobulin levels, serum and urine protein electrophoresis, electrocardiogram, and chest X-ray should be performed to determine whether the condition is systemic or localized. Localized amyloidosis is generally considered benign, and its management is guided by the patient's symptoms. The main goal is to maintain adequate airway patency and preserve the functional larynx. Treatment may vary from simple observation, more conservative endoscopic excision to radical excision by partial laryngectomy depending upon the extent of involvement of larynx. Endoscopic excision can be done either with cold knife, CO2 Laser, Microdebrider or with Coblation wands [1]. In cases with large obstructive lesions or anticipated postoperative laryngeal edema, tracheostomy may be required. Small, asymptomatic lesions should be monitored closely [15]. The review indicates that the most commonly employed treatment method was CO₂ laser, accounting for 45% of the eleven reported cases. A well-defined follow-up process is essential for effective management. The overall prognosis of Laryngeal Amyloidosis is generally good, but the probability of recurrent or residual disease is significant [16]. In the event of recurrence, repeated resections may be necessary [15]. In published case series involving ten or more patients with clearly defined localized laryngeal amyloidosis and a follow-up period exceeding five years, none required revision surgery beyond the seventh year. Therefore, it is advisable to maintain laryngological follow-up for at least seven years after the last surgical procedure in order to monitor for potential recurrences [16]. Mesolella M, and al. recommend monthly endoscopic check-ups for the first six months, then bimonthly until the end of the second year, then six-monthly for two years and yearly from the fifth year [13]. In the five-year review of recent cases, the mean follow-up duration was reported as 33 months (2.75 years), with two patients exhibiting recurrence during the first-month follow-up [1,9–14]. In our case, due to the presence of small lesions and improvement in the patient's symptoms, a strategy of close follow-up and continuous monitoring was deemed appropriate for the treatment plan. Follow-up assessments were conducted at the first, third, and sixth months, and endoscopic examinations revealed no evidence of mass progression or development of new lesions. ConclusionLaryngeal amyloidosis is an uncommon form of amyloidosis. The diagnosis of this condition, which often presents with hoarseness, relies on histopathological examination and Congo red staining. In terms of treatment, close monitoring is prioritized for small lesions, while surgery remains the main option for larger ones. Amyloidosis should be considered in the differential diagnosis of any patient presenting with hoarseness. Given the considerable likelihood of recurrent or residual disease, close and structured follow-up is essential. The first-month evaluation plays a critical role in establishing an adequate surveillance strategy. Although the duration of follow-up may vary based on individual clinical factors, we recommend a minimum of three endoscopic examinations within the first six months specifically at the first and sixth months followed by examinations at six-month intervals for a total duration of up to five years. References
|
|||||||||||||
| Keywords : Disfoni , Amiloidoz , Larinks | |||||||||||||
|