|
|||||||||
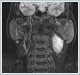
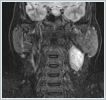
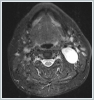
AbstractSchwannomas, also known as neurilemmomas, are tumors originating from the Schwann cells of peripheral nerve sheaths. Schwannomas originating from the cervical sympathetic plexus of the neck are extremely rare. These patients generally present with painless swelling in the neck. It is very difficult to make a definitive diagnosis preoperatively. Surgical resection is applied in treatment. Temporary or permanent Horner syndrome is an expected postoperative complication in these patients. In this paper, we discuss a case of Horner syndrome that developed following the excision of a schwannoma originating from the cervical sympathetic chain in the neck.IntroductionA schwannoma, also known as a neurilemmoma, is a tumor originating from the Schwann cells of peripheral nerve sheaths. Of all schwannomas, 25-40% are localized in the head and neck region [1,2]. Head and neck schwannomas generally originate from the cranial nerves, sympathetic chain, or branchial or cervical plexus [3]. They can originate from the cranial nerves except the optic and olfactory nerves. Among the cranial nerves, they are most commonly seen in the vestibulocochlear nerve [4]. Schwannomas originating from the sympathetic chain constitute a rare subgroup [5]. Schwannomas that develop in the neck region have a slow growth pattern and show late symptoms; therefore, their diagnosis is difficult [6.7]. The presentation complaint is usually painless swelling in the neck. Cervical schwannomas generally do not cause neurological deficits. The differential diagnosis includes carotid body tumors, glomus vagale tumors, branchial cleft cysts, malignant neck tumors, and cervical lymphadenopathies [8]. A fine-needle biopsy has very low specificity in the identification of schwannomas. The schwannomas of the sympathetic chain origin must be distinguished from carotid body and glomus vagale tumors because they may change treatment planning [9]. Surgical resection is applied in the treatment of these schwannomas. In these patients, temporary or permanent Horner syndrome is an expected complication in the postoperative period [10]. Here, we report a rare case of Horner syndrome that developed following the excision of a schwannoma originating from the cervical sympathetic chain in the neck. Case ReportA 42-year-old female patient presented to our clinic with swelling on the left side of the neck, which lasted for about one year. In the physical examination of the patient, a fixed, hard and painless swelling of approximately 3x3 cm was palpated at level 2 of the left side of the neck. Pulsation was not obtained by palpation. Other examination findings were normal. No diagnostic finding was obtained from the fine-needle aspiration biopsy. Magnetic resonance imaging (MRI) revealed a mass lesion of approximately 36x24 mm in size, originating from the carotid sheath level with higher intensity in T2-weighted sections (Figure 1 a, b).
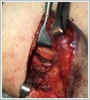
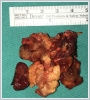
Under general anesthesia, an oblique incision of approximately 6 cm in length was made, starting from the left mastoid apex and extending 4 cm to the lower border of the mandible corpus to the mentum. The sternocleidomastoid muscle was displaced posteriorly, and the jugulocarotid sheath was reached with blunt dissection. On the left, a yellow-colored mass of approximately 5x4 cm in size was observed to originate from the cervical sympathetic chain, located posterior to the common carotid artery and the internal jugular vein and extending from the level of the common carotid artery separation to the jugular foramen superiorly and the esophagus anteriorly (Figure 3 a, b). The mass was carefully excised completely, preserving the carotid sheath content and neurovascular structures. The operation was terminated without any complications. The permanent pathological examination of the surgical specimen was reported as a schwannoma. The microscopic examination of the specimen revealed Antoni A areas consisting of palisading spindle nuclei cells and hypocellular Antoni B areas mostly comprising histiocytes and showing xanthomatous changes (Figure 4).
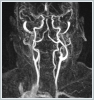
DiscussionApproximately 25-45% of extracranial schwannomas are seen in the head and neck region, with the most common localizations being the temporal bone, lateral neck, and paranasal sinuses. Among the cranial nerves, the most common is an acoustic neurinoma originating from the vestibulocochlear nerve [11]. Extracranial schwannomas can also originate from the glossopharyngeal, accessory and hypoglossal nerves. Schwannomas originating from the sympathetic chain have been reported as a rare subgroup [12]. Generally, schwannomas are more common in men between the ages of 30-60 years. However, in a study by Torosian et al., it was shown that two-thirds of extracranial schwannomas were seen in women. Our case was a 42-year-old female patient. Approximately three quarters of patients present with asymptomatic swelling in the neck [13]. It is important to differentiate a schwannoma originating from the vagal nerve and a schwannomas arising from the sympathetic chain in the preoperative period. Since the former usually occurs between the carotid artery and the internal jugular vein, it pushes both structures; thus, they move away from each other. On the other hand, a schwannoma of the sympathetic chain origin does not present with the displacement of the carotid artery and jugular vein [14]. MRA or angiography can be used for the vascular examination of the neck. In our case, no pushing of the carotid artery was observed in MRA imaging. The preoperative diagnosis of schwannomas is very difficult, and a preoperative diagnosis is not possible in most cases [12]. Biswas et al. reported that only 6% of patients diagnosed with extracranial head and neck schwannomas could be diagnosed preoperatively [15]. Our patient underwent surgery with the pre-diagnoses of glomus tumor, neurogenic tumor, and branchial cleft cyst. A fine-needle aspiration biopsy may be helpful in diagnosis. However, Kang et al. showed that a cytological examination provided conclusive results in only 20% of cases in the diagnosis of schwannoma [11]. Cytologically, the diagnosis can be made by observing Verocay bodies and spindle cells. In our case, no diagnostic finding was obtained from the fine-needle aspiration biopsy. The size, localization and vicinity of the tumor can be evaluated with imaging methods. Schwannomas have specific MRI findings, including the split fat, fascicular and target signs. In addition, the tumor is isointense to skeletal muscles in the T1 sequence, and mild heterogeneity and increased signal are observed in the T2 sequence [16]. In histological examination, the tumor shows two main features: Antoni A and Antoni B areas. Antoni A areas consist of compact, elongated spindle nuclei and cells with indistinctive cytoplasmic membranes. Verocay bodies, a typical histological feature seen in the Antoni A areas of schwannomas, are cellular clusters with eosinophilic cytoplasmic cell extensions in the middle and a surrounding palisadic arrangement of nuclei. Microcyst formations and hemosiderin are seen in Antoni B areas consisting of cells with round nuclei [17]. The treatment of schwannomas is surgery. However, preserving the integrity of the nerve from which the tumor originates poses a surgical challenge. Kang et al. reported that the cause of the neurological deficit observed in the postoperative period in surgically treated schwannoma cases was the iatrogenic injury of the area where the nerve originated or terminated [11]. In the postoperative period, Horner syndrome may occur due to direct injury to the cervical sympathetic chain during excision or indirect injury caused by traction on the sympathetic chain. While indirect injuries usually heal spontaneously over time, recovery is not expected in cases where the sympathetic trunk is injured [18]. In our case, Horner syndrome was observed temporarily in the postoperative period. During the follow-up of the patient, this finding completely improved at the postoperative sixth month. Conclusion In conclusion, cervical sympathetic chain schwannomas generally present with asymptomatic swelling in the neck, and their diagnosis is based on computed tomography, MRI and fine-needle aspiration biopsy findings. In treatment, it is recommended to excise the tumor completely. Surgery should be performed very carefully in order to prevent damage to the nerve and vital structures from which the tumor originates and to reduce postoperative complications. Patients should be aware of the risk of Horner syndrome development. References
|
|||||||||
| Keywords : Servikal sempatik zincir , schwannom , Horner Sendromu | |||||||||
|